The Science
Do You Love Big Words? Learn About Tensegrity, Mechanotransduction & SP1KE ™
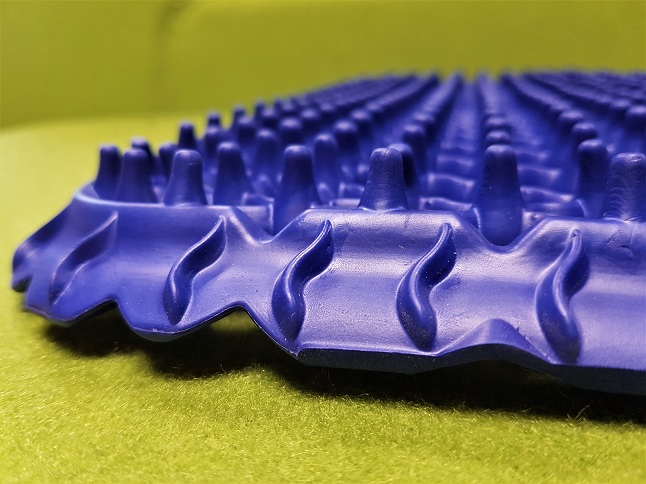
SP1KE ™ by Vigurus Technologies represents a revolutionary concept regarding the interface of human interaction and the immediate environment (i.e., sitting, standing, lying). It possesses the unique quality of adapting to any load imposed on it by the body whether it be tension, compression, shear, bending, and torsion while still maintaining its integrity. This is achieved by its patented design facilitating the proper redistribution of load three dimensionally. A closer observation of its structure provides both an appreciation and an insight into its beneficial application between the interaction of the human body and its structure.
SP1KE ™ closely mimics the interaction of the cells in tissues.
Similar to cells, the individual interactive geometric nodes are an integral part of the meshed network, each conveying valuable information heightening its function. Within the human body this role is taken up by the fibrillar protein meshwork referred to as the extracellular matrix (ECM). Interaction between cells and the ECM (cell-matrix adhesion) and between neighbouring cells (cell-cell adhesion) are fundamental for proper growth, function, and even survival of the cells (Ruoslathi and Reed, 1994, Meredith Jr and Schwartz, 1997). The receptors responsible for cell adhesion to the ECM are the integrins. These receptors function mechanically, by attaching the cell cytoskeleton to the ECM, and biochemically, by sensing whether adhesion has occurred (Humphries, 2000, Hynes, 2002). Therefore, when a force is imparted on the body, this mechanical perturbation through the relationship of the ECM, integrins and the cytoskeleton results in a biochemical response of the cell. This process alludes to the concept of mechanotransduction, which has been suggested as a key regulator of many physiological processes involved in muscle adaptation promoting a potential remodeling of the ECM. Similar to muscle, which is a highly plastic adaptable tissue to various physical activities, SP1KE ™ possesses such qualities.
The main tenant of mechanotransduction is the concept of tensegrity (Ingber, 2008b). Tensegrity is concerned with how stability is created by network structures through continuous tension (tensional tensegrity) and compression. In order to achieve this, the system itself needs to be in a state of pre-stress which is essential for maintaining mechanical stability (Ingber, 2008a). Similarly, the morphological design of SP1KE ™ is conducive for creating a state of pre-stress accounting for its sensitive and direct response counteracting any mechanical load placed on it. The magnitude of the response is in direct proportion to the load placed on SP1KE ™, whether the intensity of the load is low, medium and/or high. This functional and malleable stability is an important design feature differentiating SP1KE ™ from any other product within the market place. For instance, if SP1KE ™ is exposed to a compressive force it counteracts this by generating tension through its many nodes achieving stability by distributing and equilibrating the applied force throughout its structure.
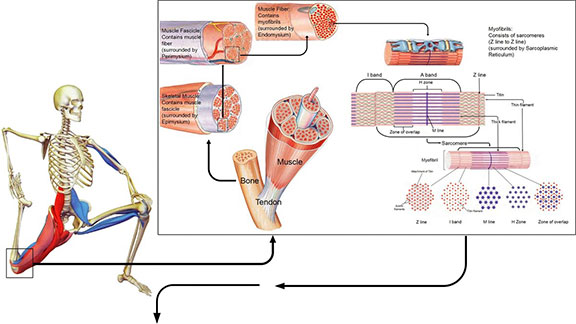
According to Ingber, our bodies are constructed of a hierarchy of systems within systems, each with its own tensional integrity such that the tensegrity at one level or size scale may itself be a tensegrity composed of smaller tension/compression elements at another level or a reduced size scale (Ingber, 2006). These prestressed hierarchies exhibit a mechanical responsiveness to a mechanical stress or load which is influenced by the magnitude of the stress itself. The relationship between the different layers of the musculoskeletal system may serve as an example of mechanotransduction and tensegrity. At the macroscopic layer, the stable vertical form of the body relative to the force of gravity is maintained between the compression bearing bones of the skeleton and the tensile pull of the muscle, tendons and ligaments (Ingber, 1998). The pre-stress arises from the balance between the contractile forces generated by the cytoskeleton of the muscle cells countered by the bone matrix’s ability to resist. Below this level, mechanical force is distributed through the myofascial and ECM connections between adjacent muscle bundles, blood vessels, and nerve tracts (Ingber, 2006).
Further down at the cellular level, balance between compression and tension of the individual muscle bundles and blood vessels is achieved between tractional forces at the parenchymal cells resisting the forces exerted by the ECMs surrounding the connective tissue cells, ensuring stability (Ingber, 2006). This rearranging at many size scales of the musculoskeletal system tries to protect it against injury due to impact and/or repetitive strain, since the molecular components that comprise the tensed ECMs and interconnected cytoskeletal elements within the adherent cells through the integrins, adjust accordingly to the mechanical load imparted on the system as a whole (Ingber, 2006, Komulainen et al., 1998, Ralphs et al., 2002). The biochemical response to a mechanical perturbation is contingent on this architectural hierarchy, which has also been expressed in terms of the importance of muscle and its ability to adapt to its immediate environment (Gans and Abbot, 1991).
As the founder and developer of Stretch Therapy and the technique of microStretching®, I have always been interested in the response of stretching intensity and its effect on inflammation and the inflammatory response. Since stretching is defined as an external and/or internal force with the magnitude of the force applied, as the intensity of the stretch, muscle stretching generates a mechanotransduction response believed to be responsible for the expression of specific genes, promoting sarcomeregenesis and the remodeling of the ECM in shortened and atrophied muscles (Martins et al., 2013). It is my belief that I anticipate that the use of SP1KE ™ in conjunction with microStretching only heightens the mechanotransduction response. This response has huge applicability for the recovery of muscle as well as to provide a therapeutic aide to individuals suffering from muscle injury, and pathological conditions associated with inflammation (i.e., osteo-arthritis).
References
GANS, C. & ABBOT, S. G. 1991. Muscle architecture in relation to function. J Biomech, 24, 53-65.
HUMPHRIES, M. J. 2000. Integrin structure. Biochem Soc Trans, 28, 311-339.
HYNES, R. O. 2002. Integrins: bidirectional, allosteric signaling machines. Cell, 110, 673-687.
INGBER, D. E. 1998. The architecture of life. Sci Amer, 278, 48-57.
INGBER, D. E. 2006. Cellular mechanotransduction: putting all the pieces together again. FASEB, 20, 811-827.
INGBER, D. E. 2008a. Tensegrity-based mechanosensing form macro to micro. Prog Biophys Mol Biol, 97, 163-179.
INGBER, D. E. 2008b. Tensegrity and mechanotransduction. J Bodyw Movem Ther, 12, 198-200.
KOMULAINEN, J., TAKALA, T. E. S., KUIPERS, H. & HESSELINK, M. K. C. 1998. The disruption of the myofibre structures in rat skeletal muscle after forced lengthening contractions. Pflugers Arch – Eur J Physiol, 117, 29-35.
MARTINS, W. R., CARVALHO, M. M., MOTA, M. R., CIPRIANO, G. F. B., MENDES, F. A. S., DINIZ, L. R., JUNIOR, G. C., CARREGARO, R. L. & DURIGAN, J. L. Q. 2013. Dicutaneous fibrolysis versus passive stretching after articular immobilisation: muscle recvoery and extracellualr matrix reodelling. OA Med Hypo, 1, 17-22.
MEREDITH JR, J. E. & SCHWARTZ, M. A. 1997. Integrins, adhesion and apoptosis. Trends Cell Biol, 7, 146-150.
RALPHS, J. R., WAGGET, A. D. & BENJAMIN, M. 2002. Actin stress fibres and cell-cell adhesion molecules in tendons: organisation in vivo and response to mechanical loading of tendon cells in vitro. Matrix Biol, 21, 67-74.
RUOSLATHI, E. & REED, J. C. 1994. Anchorage dependence, integrins, and apoptosis. Cell, 77, 477-478.